Details of the recall of Equimax LV
|
Product identification |
|
|---|---|
|
Product type |
Veterinary medicine |
|
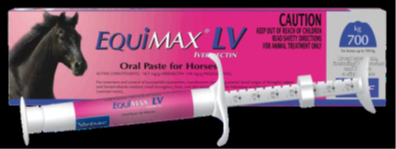
Product name |
Equimax LV |
|
ACVM registration number |
A008044 |
|
Batch number |
30603/1 |
|
Expiry date |
30 Apr 2025 |
|
Package size and description |
This product is sold in a 7.49g syringe. |
|
Distribution |
This batch has been sold at selected veterinary clinics and retail stores throughout New Zealand between January and March 2024. |
|
ACVM reference number |
NC-24-028 |
|
Notes |
This recall does not affect any other batches of this product or any other Virbac products. |
Point-of-sale notice for retailers
If you are a retailer of the product in this recall, download a copy of the point-of-sale notice and display it in your store for one month.
Equimax LV retail recall notice [PDF, 363 KB]
Consumer advice
This product should not be used. Customers should return the affected product to their place of purchase for a full refund.
No adverse event reports have been received. If you have used any of this product and have any concerns about the health of any animal treated, seek veterinary advice.
Who to contact
If you have questions, contact Virbac New Zealand Limited:
- Phone: 0800 84 72 22
- Email: enquiries@virbac.co.nz







